
Calcium gives liquid batteries a surprising boost
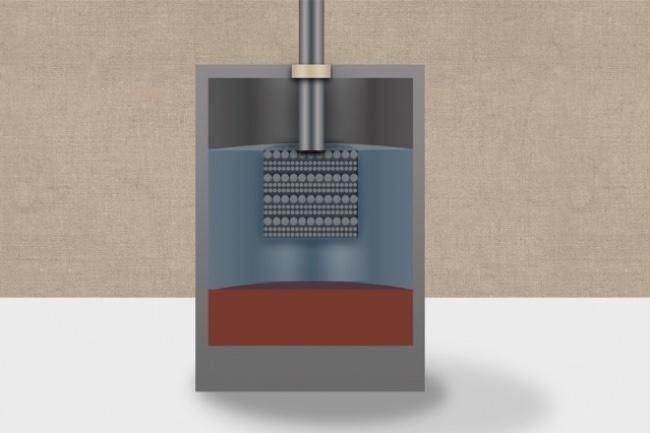
Liquid batteries, developed by MIT professor Donald Sadoway, are an exciting new battery technology that allows batteries to hold large amounts of energy for up to 12 hours, and discharge it slowly over time, making it an attractive storage option for renewable energy systems. Now Sadoway and his team have developed a new liquid battery matrix that promises to make the battery even more efficient and affordable for users. Developed by Sadoway and commercialized by Ambri, liquid power cells are unique because all the components are in a liquid state during operation. The batteries originally used magnesium as the negative electrode and antimony as the positive electrode along with a low-cost molten salt electrolyte. The new battery technology uses calcium, which is of course a fairly abundant and affordable chemical, for both the electrodes and the molten salt inside the battery. Calcium was a complex chemical to work with because it dissolves quickly in salt, making it challenging to use in a liquid battery, which requires three separate liquid layers that remain distinct while still functioning together as a battery. Calcium also has a high melting point that theoretically required the battery to operate at 900 degrees Celsius. “It was the most difficult chemistry,” said Sadonway, who is the John F. Elliott Professor of Materials Chemistry at MIT. Related: This 9,000mAh portable battery bank can recharge itself in only 18 minutes To overcome the heating issue, the team mixed magnesium with the calcium when creating the liquid electrodes. Magnesium has a much lower melting point, allowing the battery to operate at significantly lower temperatures. The team also developed a new formulation for the battery’s inner electrolyte layer, which provides the matrix for the transfer of ions between the electrodes. The new salt-based formulation uses lithium chloride and calcium chloride, and this permits ion exchange at a significantly higher rate than the previously developed liquid battery technology. The new lithium electrolyte has a second, unexpected side benefit — besides lowering the operating temperature and boosting battery output, it also helps maintain the tri-layer nature of the power cell by preventing the calcium-magnesium electrodes from dissolving in the salt. And perhaps the biggest advantage to this new liquid battery is from the supply side of the technology. Both calcium and magnesium are mined together and expensive to separate. Since these new batteries use calcium and magnesium together, producing the batteries is much more affordable. Sadoway and his team note that this new formulation is a starting point for a new field of battery technology. The team hopes this work will inspire other scientists to explore other chemical combinations that are efficient at conducting electricity and are even more affordable to produce. “The lesson here is to explore different chemistries and be ready for changing market conditions,” Sadoway says. Also watch: Raimond de Hullu’s vision for Oas1s green buildings
By Kelly Hodgkins

